In the realm of chemistry, the concept of mole measurement holds immense significance, offering a precise and quantifiable approach to understanding the composition and behavior of substances. This comprehensive guide delves into the intricacies of mole measurement, providing a clear and engaging exploration of its terminology, calculations, and applications in various chemical contexts.
From understanding the fundamental concept of “mole” to unraveling the significance of Avogadro’s number, this guide unravels the complexities of mole measurement, equipping readers with a solid foundation in this essential aspect of chemistry.
Mole Measurement Terminology
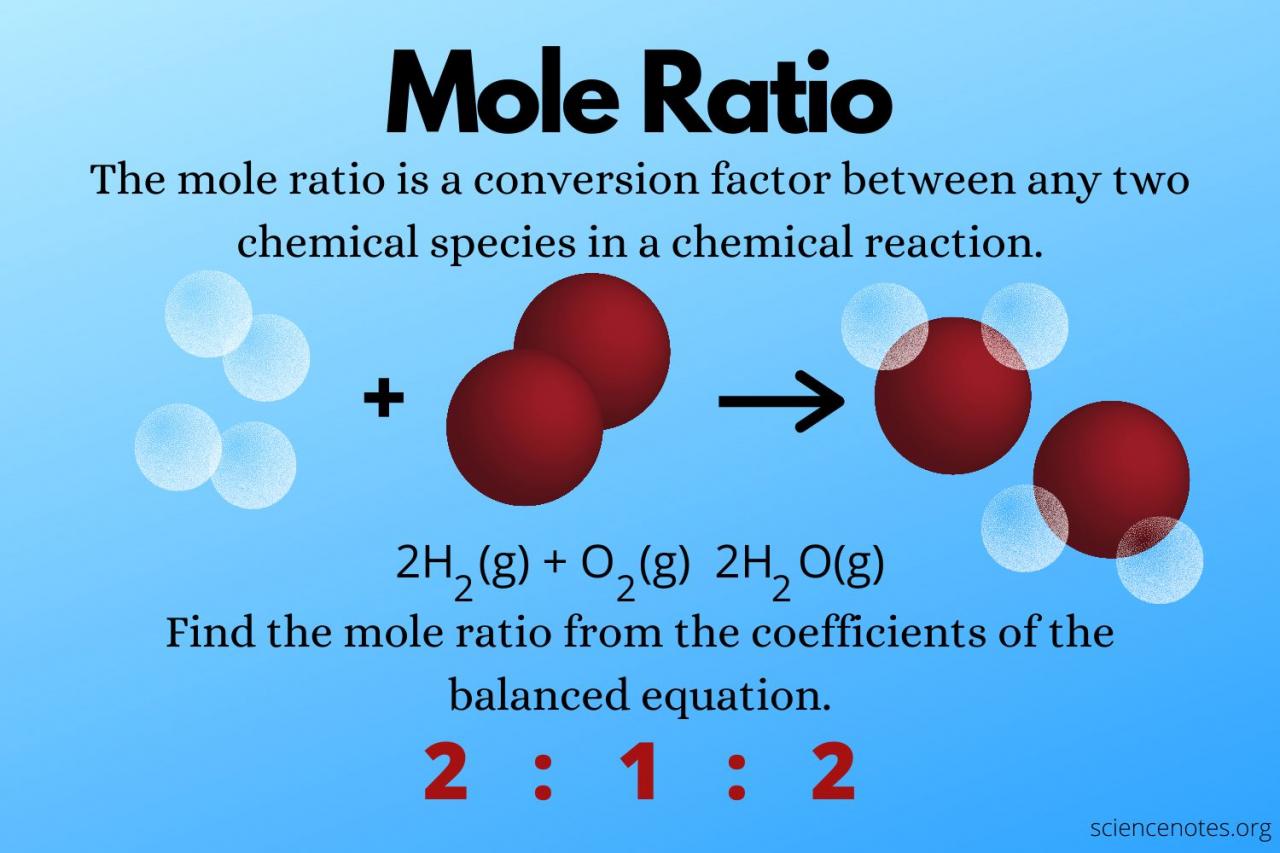
The mole is a fundamental unit in chemistry that represents a specific quantity of particles, such as atoms, molecules, or ions. It is defined as the amount of substance that contains exactly 6.02214076 x 10 23particles.
This number, known as Avogadro’s number, is a crucial concept in mole measurement. It provides a bridge between the macroscopic and microscopic scales, allowing chemists to relate the mass of a substance to the number of particles it contains.
Moles are used extensively in chemical reactions to quantify reactants and products. For example, in the reaction between hydrogen and oxygen to form water, the mole ratio of hydrogen to oxygen is 2:1. This means that for every 2 moles of hydrogen, 1 mole of oxygen is required for complete combustion.
Mole Measurement Calculations
Converting between mass and moles is a common task in chemistry. The molar mass of a substance, expressed in grams per mole (g/mol), provides the link between these two units.
To convert from mass to moles, divide the mass by the molar mass. Conversely, to convert from moles to mass, multiply the moles by the molar mass.
Stoichiometry plays a vital role in mole-based calculations. Stoichiometry involves the study of the quantitative relationships between reactants and products in chemical reactions. By using mole ratios derived from balanced chemical equations, chemists can determine the exact amount of each reactant and product involved in a reaction.
Mole Measurement in Chemical Reactions
In chemical reactions, moles are used to determine the limiting reactant, which is the reactant that is completely consumed, limiting the amount of product that can be formed.
Mole ratios also help predict product yields and reaction outcomes. By calculating the moles of reactants and products, chemists can estimate the maximum amount of product that can be obtained under specific conditions.
Mole Measurement in Solutions
Molarity is a unit of concentration that expresses the number of moles of solute per liter of solution. It is used to prepare and analyze solutions with precise concentrations.
To calculate the molarity of a solution, divide the number of moles of solute by the volume of the solution in liters.
Mole Measurement in Gas Laws
In gas laws, moles are used to relate the volume, pressure, and temperature of gases. The Ideal Gas Law combines these variables into a single equation: PV = nRT, where P is pressure, V is volume, n is the number of moles, R is the gas constant, and T is temperature.
Mole measurements are essential for determining gas properties, such as density and molar mass, and for solving problems involving gas behavior.
Final Review

Through a comprehensive exploration of mole measurement, this guide empowers individuals to navigate the intricacies of chemical reactions, solution chemistry, and gas laws with precision and confidence. By mastering the art of mole measurement, chemists and students alike gain a deeper understanding of the quantitative relationships that govern the behavior of matter, unlocking a world of possibilities in scientific research and applications.
FAQs
What is the significance of Avogadro’s number in mole measurement?
Avogadro’s number serves as a fundamental constant, representing the number of atoms, molecules, or ions present in one mole of a substance. It establishes a direct relationship between the mass and the number of particles in a given sample, facilitating precise calculations and comparisons.
How does mole measurement aid in predicting product yields in chemical reactions?
By utilizing mole ratios derived from balanced chemical equations, mole measurement enables chemists to determine the limiting reactant and predict the maximum yield of products in a given reaction. This quantitative approach ensures efficient utilization of reactants and accurate forecasting of reaction outcomes.
What is the role of molarity in solution chemistry?
Molarity, expressed as moles of solute per liter of solution, serves as a crucial parameter in solution chemistry. It quantifies the concentration of a solution, allowing for precise preparation, dilution, and analysis of solutions. Molarity plays a vital role in various chemical and biological applications.